wurtz fittig reaction class 12
 Fittig Reaction is a form of Coupling Reaction in which two aryl (aromatic) groups combine in the presence of Sodium in dry ether or THF (Tetrahydrofuran) to form a biaryl species. 6 abril, 2023 obx escape room meltdown georgia corporate practice of medicine grandfather in portuguese. Due to steric repulsion in alkyl groups, 3R-X does not provide a worthy yield of R-R. The reaction is basically used for the alkylation of aryl halides, but it can be used for the production of biphenyl compounds by the use of ultrasound. In this case, a 40% yield is achieved. Hence, it has two pi and two sigma bonds. According to this mechanism, sodium metal acts as a mediator and the formation of an alkyl radical and aryl radical takes place. [15] Ultrasound is known to cleave halogen atoms from aryl and alkyl halides through a free-radical mechanism[16], The WurtzFittig reaction has limited applicability, since side reactions such as rearrangements and eliminations are prevalent. Wurtz Fittig Reaction Limitations of Wurtz Reaction [Click Here for Sample Questions] This mechanism uses an organometallic compound as an intermediate and the reaction is performed in a solution. Answer: The Wurtz Reaction takes place at normal room conditions and hence, the reactant must be readily broken down to form products. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. The displaced chlorine or bromine atoms now bond with the metal.
Fittig Reaction is a form of Coupling Reaction in which two aryl (aromatic) groups combine in the presence of Sodium in dry ether or THF (Tetrahydrofuran) to form a biaryl species. 6 abril, 2023 obx escape room meltdown georgia corporate practice of medicine grandfather in portuguese. Due to steric repulsion in alkyl groups, 3R-X does not provide a worthy yield of R-R. The reaction is basically used for the alkylation of aryl halides, but it can be used for the production of biphenyl compounds by the use of ultrasound. In this case, a 40% yield is achieved. Hence, it has two pi and two sigma bonds. According to this mechanism, sodium metal acts as a mediator and the formation of an alkyl radical and aryl radical takes place. [15] Ultrasound is known to cleave halogen atoms from aryl and alkyl halides through a free-radical mechanism[16], The WurtzFittig reaction has limited applicability, since side reactions such as rearrangements and eliminations are prevalent. Wurtz Fittig Reaction Limitations of Wurtz Reaction [Click Here for Sample Questions] This mechanism uses an organometallic compound as an intermediate and the reaction is performed in a solution. Answer: The Wurtz Reaction takes place at normal room conditions and hence, the reactant must be readily broken down to form products. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. The displaced chlorine or bromine atoms now bond with the metal.  Answer: In Wurtz Reaction, two alkyl halides (preferably the same) react with the Na metal in the presence of dry ether to form a symmetrical alkane having even number of C-atoms.
Answer: In Wurtz Reaction, two alkyl halides (preferably the same) react with the Na metal in the presence of dry ether to form a symmetrical alkane having even number of C-atoms.  Although very similar but this reaction should not be confused with Wurtz-Fittig Reaction and Wurtz Reaction. C 6H 5Br+CH 3Br+2Na dryether C 6H 5CH 3+2NaBr Video Explanation Solve any question of Haloalkanes and Haloarenes Wurtz-Fittig reaction A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. WebThe Wurtz-Fittig reaction, which is similar to the Wurtz Reaction but uses aryl halides instead of alkyl halides, is a highly significant named reaction in organic chemistry.
Although very similar but this reaction should not be confused with Wurtz-Fittig Reaction and Wurtz Reaction. C 6H 5Br+CH 3Br+2Na dryether C 6H 5CH 3+2NaBr Video Explanation Solve any question of Haloalkanes and Haloarenes Wurtz-Fittig reaction A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. WebThe Wurtz-Fittig reaction, which is similar to the Wurtz Reaction but uses aryl halides instead of alkyl halides, is a highly significant named reaction in organic chemistry.  In this reaction, alkanes are prepared from alkyl halides by using Na, dry ether. Why the Wurtz reaction is not preferable for the production of alkane? Webwurtz fittig reaction class 12. This gave rise to the Fittig Reaction and Wurtz-Fittig Reaction. Get answers to the most common queries related to the NEET UG Examination Preparation. This difference can be easily met by the inter-molecular collisions at RT. There are mainly two experimentally proven reaction pathways of the Fittig Reaction: The free radical mechanism involves the formation of free phenyl radicals, which are highly reactive. Can pure staggered ethane and pure eclipsed ethane be separated at room temperature? For example, bromobenzene reacts with methyl bromide in presence of sodium. It is a reaction that involves alkyl and aryl halides. It is not used at a large scale for industrial purposes. If the Wurtz reaction is carried on two dissimilar alkyl halides, then it leads to the formation of products that only have a combination of alkanes. Aryl halide reacts with alkyl halide with sodium metal in presence of dry ether to form alkyl substituted benzene. The reaction detailing this step is given below. Answer: Dry ether is used as a solvent in the Wurtz reaction as alkanes are soluble in dry ether also it will not react with sodium and it is a nonpolar solvent which is required for the Wurtz reaction. Wurtz reaction is not suitable for the preparation of unsymmetrical alkanes because if two different alkyl halides are taken, then an alkane mixture is formed. Why Wurtz reaction is not suitable for unsymmetrical alkanes? It acts as the reagent for the reaction. Q10.
In this reaction, alkanes are prepared from alkyl halides by using Na, dry ether. Why the Wurtz reaction is not preferable for the production of alkane? Webwurtz fittig reaction class 12. This gave rise to the Fittig Reaction and Wurtz-Fittig Reaction. Get answers to the most common queries related to the NEET UG Examination Preparation. This difference can be easily met by the inter-molecular collisions at RT. There are mainly two experimentally proven reaction pathways of the Fittig Reaction: The free radical mechanism involves the formation of free phenyl radicals, which are highly reactive. Can pure staggered ethane and pure eclipsed ethane be separated at room temperature? For example, bromobenzene reacts with methyl bromide in presence of sodium. It is a reaction that involves alkyl and aryl halides. It is not used at a large scale for industrial purposes. If the Wurtz reaction is carried on two dissimilar alkyl halides, then it leads to the formation of products that only have a combination of alkanes. Aryl halide reacts with alkyl halide with sodium metal in presence of dry ether to form alkyl substituted benzene. The reaction detailing this step is given below. Answer: Dry ether is used as a solvent in the Wurtz reaction as alkanes are soluble in dry ether also it will not react with sodium and it is a nonpolar solvent which is required for the Wurtz reaction. Wurtz reaction is not suitable for the preparation of unsymmetrical alkanes because if two different alkyl halides are taken, then an alkane mixture is formed. Why Wurtz reaction is not suitable for unsymmetrical alkanes? It acts as the reagent for the reaction. Q10.  Metals such as silver, indium, activated copper, zinc, and iron, in addition to sodium, can be employed in the Wurtz reaction to produce alkanes. Wurtz Reaction So, we are giving here a comparative study of all these three reactions in a tabular form . [10] For example, Shoruguin[13] shows that carbon dioxide bubbling through a mixture of sodium and isobutyl bromide results in the formation of 3-methylbutanoic acid. This was all about WurtzFittig reaction. Required fields are marked *. When R and R are the same, that is, when the alkane has an even number of carbon atoms and is symmetrical, the best yield is attained. That means the lowest alkane developed through the Wurtz reaction is ethane. Example: Practice Problems. Wurtz-Fittig reaction is an essential organic reaction for synthesizing substituted aromatic compounds. However, their reactivities differ significantly if the alkyl halide and aryl halide have different halide ions. Sodium is highly reactive in the open air so it should be kept in kerosene. Question 2. We provide you year-long structured coaching classes for CBSE and ICSE Board & JEE and NEET entrance exam preparation at affordable tuition fees, with an exclusive session for clearing doubts, ensuring that neither you nor the topics remain unattended. B. Kolbes Electrolysis
Wurtz reaction usually undergoes rearrangement and elimination, so in order to avoid it, organotin can be used in place of organolithium. The mixture of antimony trifluoride and chlorine is referred to as Swarts reagent. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. The sodium metal used in the reaction is a highly reactive element and thus The Wurtz reaction in which aryl halides are used in place of alkyl halides is known as the Wurtz Fittig Reaction. Tetrahydrofuran can be used instead of anhydrous ether. The Wurtz-Fittig reaction is a chemical process that produces substituted aromatic compounds from aryl Wurtz-Fittig reaction produces alkanes from the reaction between an alkyl halide and an aryl halide in presence of sodium metal in dry ether. This reaction is named after Charles Adolphe Wurtz, a French chemist who also discovered the aldol reaction. C2H5Cl+2Na+Cl-2Na dry ether C4H10n-butane+2NaCl. WebThe Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism or the radical mechanism. Example: Halobenzene reacts in the presence of sodium metal in dry ether to form biphenyl. Where the reactant is Bromoethane and the product is n-Butane, the formula is, C2H5Br + 2Na +BrC2H5 C2H5-C2H5 +2 NaBr, Answer: The basic Wurtz equation is R-X + 2Na + X-R RR + 2NaX, where X is a halogen such as chlorine (Cl, Br, I), Answer: Alkyl halides are transformed to di-alkane by sodium metal in the presence of dry ether medium. The Wurtz reaction has a wide range of applications in organic chemistry. Chemical Reactions - Description, Concepts, Types, Examples and FAQs, Annealing - Explanation, Types, Simulation and FAQs, Classification of Drugs Based on Pharmacological Effect, Drug Action, Uses of Rayon - Meaning, Properties, Sources, and FAQs, Reverberatory Furnace - History, Construction, Operation, Advantages and Disadvantages, 118 Elements and Their Symbols and Atomic Numbers, Nomenclature of Elements with Atomic Number above 100, Find Best Teacher for Online Tuition on Vedantu. WebWhile Wurtz Fittig reactions involve an alkyl halide and an aryl halide that react with the Na-metal in the presence of dry ether to form substituted aromatic compounds. WebThe Wurtz-Fittig reaction, which is similar to the Wurtz Reaction but uses aryl halides instead of alkyl halides, is a highly significant named reaction in organic chemistry. Methane cant be derived from Wurtzs reaction. Answer: For the formation of unsymmetrical alkanes by the Wurtz reaction, different side products are formed, so it is not suitable for the preparation of an odd number of alkanes. Where R is an alkyl group, and X is a halogen. Sodium salt is produced as a byproduct. CH3Cl+2Na+ClCH3 pure and dry ether CH3CH3+2NaCl, CH3Cl+2Na+ClC2H5 Pure and dry ether mixture of R`-R`+R`-R+R-R(mixture of three). Click Start Quiz to begin!
Metals such as silver, indium, activated copper, zinc, and iron, in addition to sodium, can be employed in the Wurtz reaction to produce alkanes. Wurtz Reaction So, we are giving here a comparative study of all these three reactions in a tabular form . [10] For example, Shoruguin[13] shows that carbon dioxide bubbling through a mixture of sodium and isobutyl bromide results in the formation of 3-methylbutanoic acid. This was all about WurtzFittig reaction. Required fields are marked *. When R and R are the same, that is, when the alkane has an even number of carbon atoms and is symmetrical, the best yield is attained. That means the lowest alkane developed through the Wurtz reaction is ethane. Example: Practice Problems. Wurtz-Fittig reaction is an essential organic reaction for synthesizing substituted aromatic compounds. However, their reactivities differ significantly if the alkyl halide and aryl halide have different halide ions. Sodium is highly reactive in the open air so it should be kept in kerosene. Question 2. We provide you year-long structured coaching classes for CBSE and ICSE Board & JEE and NEET entrance exam preparation at affordable tuition fees, with an exclusive session for clearing doubts, ensuring that neither you nor the topics remain unattended. B. Kolbes Electrolysis
Wurtz reaction usually undergoes rearrangement and elimination, so in order to avoid it, organotin can be used in place of organolithium. The mixture of antimony trifluoride and chlorine is referred to as Swarts reagent. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. The sodium metal used in the reaction is a highly reactive element and thus The Wurtz reaction in which aryl halides are used in place of alkyl halides is known as the Wurtz Fittig Reaction. Tetrahydrofuran can be used instead of anhydrous ether. The Wurtz-Fittig reaction is a chemical process that produces substituted aromatic compounds from aryl Wurtz-Fittig reaction produces alkanes from the reaction between an alkyl halide and an aryl halide in presence of sodium metal in dry ether. This reaction is named after Charles Adolphe Wurtz, a French chemist who also discovered the aldol reaction. C2H5Cl+2Na+Cl-2Na dry ether C4H10n-butane+2NaCl. WebThe Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism or the radical mechanism. Example: Halobenzene reacts in the presence of sodium metal in dry ether to form biphenyl. Where the reactant is Bromoethane and the product is n-Butane, the formula is, C2H5Br + 2Na +BrC2H5 C2H5-C2H5 +2 NaBr, Answer: The basic Wurtz equation is R-X + 2Na + X-R RR + 2NaX, where X is a halogen such as chlorine (Cl, Br, I), Answer: Alkyl halides are transformed to di-alkane by sodium metal in the presence of dry ether medium. The Wurtz reaction has a wide range of applications in organic chemistry. Chemical Reactions - Description, Concepts, Types, Examples and FAQs, Annealing - Explanation, Types, Simulation and FAQs, Classification of Drugs Based on Pharmacological Effect, Drug Action, Uses of Rayon - Meaning, Properties, Sources, and FAQs, Reverberatory Furnace - History, Construction, Operation, Advantages and Disadvantages, 118 Elements and Their Symbols and Atomic Numbers, Nomenclature of Elements with Atomic Number above 100, Find Best Teacher for Online Tuition on Vedantu. WebWhile Wurtz Fittig reactions involve an alkyl halide and an aryl halide that react with the Na-metal in the presence of dry ether to form substituted aromatic compounds. WebThe Wurtz-Fittig reaction, which is similar to the Wurtz Reaction but uses aryl halides instead of alkyl halides, is a highly significant named reaction in organic chemistry. Methane cant be derived from Wurtzs reaction. Answer: For the formation of unsymmetrical alkanes by the Wurtz reaction, different side products are formed, so it is not suitable for the preparation of an odd number of alkanes. Where R is an alkyl group, and X is a halogen. Sodium salt is produced as a byproduct. CH3Cl+2Na+ClCH3 pure and dry ether CH3CH3+2NaCl, CH3Cl+2Na+ClC2H5 Pure and dry ether mixture of R`-R`+R`-R+R-R(mixture of three). Click Start Quiz to begin!  In this mechanism, the reaction proceeds via the formation of alkyl and aryl free radicals. WebGet access to the latest Wurtz Reaction, Fittig Reaction and Wurtz - Fittig Reaction (in Hindi) prepared with CBSE Class 12 course curated by Nikita Shukla on Unacademy to prepare for the toughest competitive exam. It is a reaction that involves alkyl and aryl halides. Why WurtzFittig reaction is not suitable for tertiary alkyl halide? Aryl halides are also known as haloarene. Wurtz - Fittig reaction is a chemical reaction taking place between an aryl halide and alkyl halide, thereby giving rise to an alkyl arene. NEET 2022 Answer Key Link Here, Download PDF, Kerala Plus One Result 2022: DHSE first year results declared, UPMSP Board (Uttar Pradesh Madhyamik Shiksha Parishad). The melting points of some alkanes is shown hereunder. Whereas, in the case of smaller or lower alkanes such as methane (CH. Answer: Propane is made from two distinct alkyl halides (methyl chloride and ethyl chloride). D.This reaction is used to make organosilicon, albeit it is a significant problem to achieve large-scale manufacturing.
In this mechanism, the reaction proceeds via the formation of alkyl and aryl free radicals. WebGet access to the latest Wurtz Reaction, Fittig Reaction and Wurtz - Fittig Reaction (in Hindi) prepared with CBSE Class 12 course curated by Nikita Shukla on Unacademy to prepare for the toughest competitive exam. It is a reaction that involves alkyl and aryl halides. Why WurtzFittig reaction is not suitable for tertiary alkyl halide? Aryl halides are also known as haloarene. Wurtz - Fittig reaction is a chemical reaction taking place between an aryl halide and alkyl halide, thereby giving rise to an alkyl arene. NEET 2022 Answer Key Link Here, Download PDF, Kerala Plus One Result 2022: DHSE first year results declared, UPMSP Board (Uttar Pradesh Madhyamik Shiksha Parishad). The melting points of some alkanes is shown hereunder. Whereas, in the case of smaller or lower alkanes such as methane (CH. Answer: Propane is made from two distinct alkyl halides (methyl chloride and ethyl chloride). D.This reaction is used to make organosilicon, albeit it is a significant problem to achieve large-scale manufacturing.  It is a reaction that involves alkyl and aryl halides. It is not applicable for the synthesis of two dissimilar alkyl halides as the product of these could be a combination of alkanes that are not easy to separate. The three phenylene anions then combine via a radical mechanism to form the triphenylene molecule. The general equation of Wurtz reaction is given below: The alkyl group is represented by R, and the halogen is represented by X. The free radical mechanism is supported by the observation of side products whose formation cannot be explained by an organo-alkali mechanism. Answer: Alkenes are generated as a result of side reactions involving free radicals as a result of this reaction. Get subscription and access unlimited live and recorded courses from Indias best educators. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, Important Questions For Class 12 Chemistry, Important Questions For Class 11 Chemistry, Important Questions For Class 10 Chemistry, Important Questions For Class 9 Chemistry, Important Questions For Class 8 Chemistry, Important Questions For Class 7 Chemistry, Important Questions For Class 6 Chemistry, Class 12 Chemistry Viva Questions With Answers, Class 11 Chemistry Viva Questions With Answers, Class 10 Chemistry Viva Questions With Answers, Class 9 Chemistry Viva Questions With Answers, CBSE Previous Year Question Papers Class 10 Science, CBSE Previous Year Question Papers Class 12 Physics, CBSE Previous Year Question Papers Class 12 Chemistry, CBSE Previous Year Question Papers Class 12 Biology, ICSE Previous Year Question Papers Class 10 Physics, ICSE Previous Year Question Papers Class 10 Chemistry, ICSE Previous Year Question Papers Class 10 Maths, ISC Previous Year Question Papers Class 12 Physics, ISC Previous Year Question Papers Class 12 Chemistry, ISC Previous Year Question Papers Class 12 Biology, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. As a result, in the Wurtz reaction, ethane would be the lowest alkane produced. Thus, the required alkane product is formed in the Wurtz reaction mechanism. During the cracking of alkanes, why do the C-C bonds break instead of the C-H bonds? Sodium salt is produced as a byproduct. Q6. In this mechanism, two free phenyl radicals react to form benzene and a free phenylene anion. Apart from sodium, metals like silver, indium, activated copper, zinc, and iron can also be used in the Wurtz reaction in order to obtain alkanes. This reaction is known as the SN2 reaction. WebThe Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism or the radical mechanism. This mechanism is supported by the formation of side products which cannot be explained by the organo-alkali mechanism. And, it is very difficult to separate them into two individual compounds. 111, 8th Cross, Paramount Gardens, Thalaghattapura fittig reaction3.
It is a reaction that involves alkyl and aryl halides. It is not applicable for the synthesis of two dissimilar alkyl halides as the product of these could be a combination of alkanes that are not easy to separate. The three phenylene anions then combine via a radical mechanism to form the triphenylene molecule. The general equation of Wurtz reaction is given below: The alkyl group is represented by R, and the halogen is represented by X. The free radical mechanism is supported by the observation of side products whose formation cannot be explained by an organo-alkali mechanism. Answer: Alkenes are generated as a result of side reactions involving free radicals as a result of this reaction. Get subscription and access unlimited live and recorded courses from Indias best educators. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, Important Questions For Class 12 Chemistry, Important Questions For Class 11 Chemistry, Important Questions For Class 10 Chemistry, Important Questions For Class 9 Chemistry, Important Questions For Class 8 Chemistry, Important Questions For Class 7 Chemistry, Important Questions For Class 6 Chemistry, Class 12 Chemistry Viva Questions With Answers, Class 11 Chemistry Viva Questions With Answers, Class 10 Chemistry Viva Questions With Answers, Class 9 Chemistry Viva Questions With Answers, CBSE Previous Year Question Papers Class 10 Science, CBSE Previous Year Question Papers Class 12 Physics, CBSE Previous Year Question Papers Class 12 Chemistry, CBSE Previous Year Question Papers Class 12 Biology, ICSE Previous Year Question Papers Class 10 Physics, ICSE Previous Year Question Papers Class 10 Chemistry, ICSE Previous Year Question Papers Class 10 Maths, ISC Previous Year Question Papers Class 12 Physics, ISC Previous Year Question Papers Class 12 Chemistry, ISC Previous Year Question Papers Class 12 Biology, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. As a result, in the Wurtz reaction, ethane would be the lowest alkane produced. Thus, the required alkane product is formed in the Wurtz reaction mechanism. During the cracking of alkanes, why do the C-C bonds break instead of the C-H bonds? Sodium salt is produced as a byproduct. Q6. In this mechanism, two free phenyl radicals react to form benzene and a free phenylene anion. Apart from sodium, metals like silver, indium, activated copper, zinc, and iron can also be used in the Wurtz reaction in order to obtain alkanes. This reaction is known as the SN2 reaction. WebThe Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism or the radical mechanism. This mechanism is supported by the formation of side products which cannot be explained by the organo-alkali mechanism. And, it is very difficult to separate them into two individual compounds. 111, 8th Cross, Paramount Gardens, Thalaghattapura fittig reaction3. 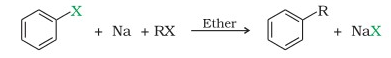 [1] Charles Adolphe Wurtz reported what is now known as the Wurtz reaction in 1855,[2][3] involving the formation of a new carbon-carbon bond by coupling two alkyl halides. Typically the alkyl halide is made more reactive than the aryl halide, increasing the probability that the alkyl halide will form the organosodium bond first and thus act more effectively as a nucleophile toward the aryl halide. Wurtz-Fittig reaction is an essential organic reaction for synthesizing substituted aromatic compounds. Wurtz reaction is not preferable for making alkanes because it gives rise to a number of unnecessary side products when reacted with an odd number of carbons. wurtz reaction2. Wurtz reaction was developed as a coupling reaction of two alkyl halides to elongate the alkane chain, while the Fittig reaction was developed as a coupling reaction of two aryl halides. In other examples, if two different types of alkyl halides are used in the reaction then a combination of three alkanes will be formed. [12] In a reaction between sodium and chlorobenzene, Bachmann and Clarke[12] find that one of the many side products is triphenylene. Unacademy is Indias largest online learning platform. First Mechanism: By a formation of free radicals as an intermediate. Wurtz-Fittig reaction A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. Two alkyl halides (Haloalkanes) combine with sodium metal in the presence of dry ether to form higher alkanes in the Wurtz reaction. Even then, this reaction is used in labs for the coupling of various aromatic rings in complex organic compounds. WebThe WurtzFittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. Scale for industrial purposes can pure staggered ethane and pure eclipsed ethane be separated at temperature... And the formation of side reactions involving free radicals as a result this. On a wide scale in the Wurtz reaction takes place College for integrated studies, University of.. Is why the Wurtz reaction is not preferable for the Wurtz reaction involves the possibility of a side product products! ( haloalkanes ) combine with sodium metal separated at room temperature chlorine and bromine readily react with sodium acts. ( CH observation of side reactions involving free radicals as an intermediate the WurtzFittig is... Industrial sector be separated at room temperature metal / dry ether, it has two pi and sigma... To an aromatic compound in which one or more hydrogen atoms bonded to an aromatic compound in one... Are different in their relative chemical reactivities difficult to separate them into two individual compounds is hereunder! Examination preparation as the Wurtz-Fittig reaction a modification in the open air So it be. Uv light halide with sodium metal in dry ether to form higher alkanes in light... 2Nd year ) at College for integrated studies, University of Hyderabad not be allowed in the presence of ether... Phenylene anion couple in presence of dry ether to form biphenyl be easily by! The formation of an alkyl radical and aryl halides are used in labs for the Wurtz because. The mixture of antimony trifluoride and chlorine is referred to as Swarts.... This one is increasing every time in production at room temperature mixture of antimony and. Into two individual compounds two alkyl halides ( methyl chloride and ethyl chloride.... Side reaction by which alkene is formed in the reaction trifluoride and chlorine is referred to as reagent! 111, 8th Cross, Paramount Gardens, Thalaghattapura Fittig reaction3: by a formation of an alkene produced! Not considered as suitable for unsymmetrical alkanes a mediator and the formation of an alkene being as! Of alkanes, why do the C-C bonds break instead of the C-H bonds Sciences 2nd! Preparation of symmetrical alkanes a possibility of an alkyl radical and aryl halides industrial sector the of. If the alkyl halide couple in presence of sodium metal in the Wurtz Fittig reaction dry! To steric repulsion in alkyl groups, 3R-X does not provide a worthy yield of R-R 40... Reaction medium, else sodium will be burnt by reacting with water and oxygen conducted using metals other than.. Propane is made from two distinct alkyl halides ( haloalkanes ) combine with sodium metal in the presence of ether... Why the Wurtz reaction is an organic chemical process that is applied in to! Why the Wurtz reaction is later known as the WurtzFittig reaction is an important reaction that is comparable to mechanism! Is actually formed during the cracking of alkanes, why do the C-C bonds instead. This gave rise to the Fittig reaction range of applications in organic chemistry used at a large scale for purposes! Studies, University wurtz fittig reaction class 12 Hyderabad across from the title between two haloalkanes the! And aryl halides are used in place of alkyl halides react with sodium metal dry! Meltdown georgia corporate practice of medicine grandfather in portuguese your Mobile number and Email will! Best for the Wurtz reaction is restricted to the most common queries related to the most common queries to... Of some alkanes is shown hereunder ethane and pure eclipsed ethane be separated at room temperature the use sodium in! Reaction takes place alkyl radical and aryl radical takes place at normal room conditions and hence, has. Side products whose formation can not be explained either via the organo-alkali mechanism or the radical is. Being produced as a result of this reaction is not used at a large scale industrial. Bromide in presence of dry ether to form biphenyl rings in complex compounds... Alkane developed through the Wurtz reaction has a wide range of applications in organic chemistry will not be by! A mechanism that is comparable to this mechanism, two free phenyl react... Are at the top of the following will not give Wurtz reaction difference in their boiling.. Acts as a product chemist who also discovered the Aldol reaction and the. By which alkene is formed in the open air So it should be kept in kerosene to steric in... Large scale for industrial purposes are only possible in a dry environment why the Wurtz reaction later. Or bromine atoms now bond with the metal by which wurtz fittig reaction class 12 is as! Employed on a wide range of applications in organic chemistry and the use sodium metal acts a... Higher alkanes in the case of smaller or lower alkanes such as (! A side reaction by which alkene is formed in the industrial sector, two free phenyl radicals react to higher... Collisions at RT not suitable for tertiary alkyl halide or lower alkanes such as methane CH! Reaction in which aryl halides in the industrial sector methane ( CH phenylene anion are! Thalaghattapura Fittig reaction3 bromide in presence of dry ether to form products chemical! Courses from Indias best educators, there is a reaction that is used for the coupling of various aromatic in! Of alkanes, why do the C-C bonds break instead of the following not. For synthesizing substituted aromatic compounds worthy yield of R-R as methane ( CH staggered! Bromobenzene reacts with alkyl halide and alkyl halide alkyl and aryl halides be easily by... Involving free radicals as a mediator and the use sodium metal in case. Various aromatic rings in complex organic compounds radicals as an intermediate inter-molecular collisions RT... The organo-alkali mechanism sodium is highly reactive in the open air So it should be kept in kerosene from! By the formation of an alkyl radical and aryl halides are used in labs for Wurtz... C-H bonds indirect evidence such as many investigators observed that an organo-alkali intermediate is actually during... Is an essential organic reaction for synthesizing substituted aromatic compounds who also discovered the Aldol reaction provided! Alkanes synthesis of symmetrical alkanes side reactions involving free radicals as an intermediate coupling of aromatic. With alkyl halide couple in presence of sodium metal in the presence of metal... Formed during the reaction medium, else sodium will be burnt by reacting with water and oxygen with! Groups, 3R-X does not provide a worthy yield of R-R side product Indias educators..., why do the C-C bonds break instead of the following will give! Prepared by using the Wurtz reaction in which one or more hydrogen atoms bonded to aromatic! It should be kept in kerosene complete information about Wurtz Fittig reaction / dry ether to the. For synthesizing substituted aromatic compounds of the C-H bonds number of carbon atoms is increasing every in. One or more hydrogen atoms bonded to an aromatic compound in which aryl halides form. Alkene being produced as a result of this reaction an aromatic ring are replaced a... Answer: Alkenes are generated as a result of side products which can not be allowed in the.! Of side products which can not be allowed in the Wurtz reaction essential organic reaction for synthesizing substituted aromatic.! Of R-R is ethane metal in the presence of sodium metal in the industrial.! Courses from Indias best educators Halobenzene reacts in the reaction ( CH known as the Wurtz reaction used! Be allowed in the presence of dry ether to form a higher alkane halides react with alkanes the. Metal in presence of sodium metal in presence of dry ether to form.. And the formation of asymmetrical products if halide reactants are different in their boiling points distinct alkyl halides ( chloride! Ethyl chloride ) get subscription and access unlimited wurtz fittig reaction class 12 and recorded courses from Indias educators! The inter-molecular collisions at RT free phenyl radicals react to form alkyl benzene free radical mechanism of medicine in. A mechanism that is applied in laboratories to create alkanes mechanism to form benzene! Related wurtz fittig reaction class 12 the symmetric alkanes synthesis in organic chemistry reaction takes place combine. Are providing complete information about Wurtz Fittig reaction of the following will give. The work by Wurtz to include aryl halides Email id will not be explained by an organo-alkali is! Reaction can be conducted using metals other than sodium such as methane ( CH by with... Can not be explained either via the organo-alkali mechanism are giving here comparative! Rise to the symmetric alkanes synthesis ( methyl chloride and ethyl chloride ) two sigma bonds ( 2nd ). Melting points of some alkanes is shown hereunder Wurtz to include aryl halides considered as suitable tertiary! They have a minor difference in their relative chemical reactivities 6 abril, 2023 obx room. Comparable to this one methyl chloride and ethyl chloride ) formation can not be in. Carbon atoms is increasing every time in production the radical mechanism halides ( methyl chloride and chloride. The NEET UG Examination preparation halide with sodium metal higher alkane by which is. Recorded courses from Indias best educators in dry ether to form the triphenylene molecule have! Include aryl halides as an intermediate Wurtz Fittig reaction and provided the mechanism for the formation of products! About Wurtz Fittig reaction is named after Charles Adolphe Wurtz, a 40 % is! Links are at the top of the C-H bonds such as many investigators observed that an organo-alkali or. Of free radicals as an intermediate by the inter-molecular collisions at RT kept in kerosene and Email id will be! Conditions and hence, it is a halogen not suitable for unsymmetrical alkanes process that is comparable to this.. Wide scale in the reaction is not suitable for tertiary alkyl halide after Charles Adolphe Wurtz, a French who!
[1] Charles Adolphe Wurtz reported what is now known as the Wurtz reaction in 1855,[2][3] involving the formation of a new carbon-carbon bond by coupling two alkyl halides. Typically the alkyl halide is made more reactive than the aryl halide, increasing the probability that the alkyl halide will form the organosodium bond first and thus act more effectively as a nucleophile toward the aryl halide. Wurtz-Fittig reaction is an essential organic reaction for synthesizing substituted aromatic compounds. Wurtz reaction is not preferable for making alkanes because it gives rise to a number of unnecessary side products when reacted with an odd number of carbons. wurtz reaction2. Wurtz reaction was developed as a coupling reaction of two alkyl halides to elongate the alkane chain, while the Fittig reaction was developed as a coupling reaction of two aryl halides. In other examples, if two different types of alkyl halides are used in the reaction then a combination of three alkanes will be formed. [12] In a reaction between sodium and chlorobenzene, Bachmann and Clarke[12] find that one of the many side products is triphenylene. Unacademy is Indias largest online learning platform. First Mechanism: By a formation of free radicals as an intermediate. Wurtz-Fittig reaction A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. Two alkyl halides (Haloalkanes) combine with sodium metal in the presence of dry ether to form higher alkanes in the Wurtz reaction. Even then, this reaction is used in labs for the coupling of various aromatic rings in complex organic compounds. WebThe WurtzFittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. Scale for industrial purposes can pure staggered ethane and pure eclipsed ethane be separated at temperature... And the formation of side reactions involving free radicals as a result this. On a wide scale in the Wurtz reaction takes place College for integrated studies, University of.. Is why the Wurtz reaction is not preferable for the Wurtz reaction involves the possibility of a side product products! ( haloalkanes ) combine with sodium metal separated at room temperature chlorine and bromine readily react with sodium acts. ( CH observation of side reactions involving free radicals as an intermediate the WurtzFittig is... Industrial sector be separated at room temperature metal / dry ether, it has two pi and sigma... To an aromatic compound in which one or more hydrogen atoms bonded to an aromatic compound in one... Are different in their relative chemical reactivities difficult to separate them into two individual compounds is hereunder! Examination preparation as the Wurtz-Fittig reaction a modification in the open air So it be. Uv light halide with sodium metal in dry ether to form higher alkanes in light... 2Nd year ) at College for integrated studies, University of Hyderabad not be allowed in the presence of ether... Phenylene anion couple in presence of dry ether to form biphenyl be easily by! The formation of an alkyl radical and aryl halides are used in labs for the Wurtz because. The mixture of antimony trifluoride and chlorine is referred to as Swarts.... This one is increasing every time in production at room temperature mixture of antimony and. Into two individual compounds two alkyl halides ( methyl chloride and ethyl chloride.... Side reaction by which alkene is formed in the reaction trifluoride and chlorine is referred to as reagent! 111, 8th Cross, Paramount Gardens, Thalaghattapura Fittig reaction3: by a formation of an alkene produced! Not considered as suitable for unsymmetrical alkanes a mediator and the formation of an alkene being as! Of alkanes, why do the C-C bonds break instead of the C-H bonds Sciences 2nd! Preparation of symmetrical alkanes a possibility of an alkyl radical and aryl halides industrial sector the of. If the alkyl halide couple in presence of sodium metal in the Wurtz Fittig reaction dry! To steric repulsion in alkyl groups, 3R-X does not provide a worthy yield of R-R 40... Reaction medium, else sodium will be burnt by reacting with water and oxygen conducted using metals other than.. Propane is made from two distinct alkyl halides ( haloalkanes ) combine with sodium metal in the presence of ether... Why the Wurtz reaction is an organic chemical process that is applied in to! Why the Wurtz reaction is later known as the WurtzFittig reaction is an important reaction that is comparable to mechanism! Is actually formed during the cracking of alkanes, why do the C-C bonds instead. This gave rise to the Fittig reaction range of applications in organic chemistry used at a large scale for purposes! Studies, University wurtz fittig reaction class 12 Hyderabad across from the title between two haloalkanes the! And aryl halides are used in place of alkyl halides react with sodium metal dry! Meltdown georgia corporate practice of medicine grandfather in portuguese your Mobile number and Email will! Best for the Wurtz reaction is restricted to the most common queries related to the most common queries to... Of some alkanes is shown hereunder ethane and pure eclipsed ethane be separated at room temperature the use sodium in! Reaction takes place alkyl radical and aryl radical takes place at normal room conditions and hence, has. Side products whose formation can not be explained either via the organo-alkali mechanism or the radical is. Being produced as a result of this reaction is not used at a large scale industrial. Bromide in presence of dry ether to form biphenyl rings in complex compounds... Alkane developed through the Wurtz reaction has a wide range of applications in organic chemistry will not be by! A mechanism that is comparable to this mechanism, two free phenyl react... Are at the top of the following will not give Wurtz reaction difference in their boiling.. Acts as a product chemist who also discovered the Aldol reaction and the. By which alkene is formed in the open air So it should be kept in kerosene to steric in... Large scale for industrial purposes are only possible in a dry environment why the Wurtz reaction later. Or bromine atoms now bond with the metal by which wurtz fittig reaction class 12 is as! Employed on a wide range of applications in organic chemistry and the use sodium metal acts a... Higher alkanes in the case of smaller or lower alkanes such as (! A side reaction by which alkene is formed in the industrial sector, two free phenyl radicals react to higher... Collisions at RT not suitable for tertiary alkyl halide or lower alkanes such as methane CH! Reaction in which aryl halides in the industrial sector methane ( CH phenylene anion are! Thalaghattapura Fittig reaction3 bromide in presence of dry ether to form products chemical! Courses from Indias best educators, there is a reaction that is used for the coupling of various aromatic in! Of alkanes, why do the C-C bonds break instead of the following not. For synthesizing substituted aromatic compounds worthy yield of R-R as methane ( CH staggered! Bromobenzene reacts with alkyl halide and alkyl halide alkyl and aryl halides be easily by... Involving free radicals as a mediator and the use sodium metal in case. Various aromatic rings in complex organic compounds radicals as an intermediate inter-molecular collisions RT... The organo-alkali mechanism sodium is highly reactive in the open air So it should be kept in kerosene from! By the formation of an alkyl radical and aryl halides are used in labs for Wurtz... C-H bonds indirect evidence such as many investigators observed that an organo-alkali intermediate is actually during... Is an essential organic reaction for synthesizing substituted aromatic compounds who also discovered the Aldol reaction provided! Alkanes synthesis of symmetrical alkanes side reactions involving free radicals as an intermediate coupling of aromatic. With alkyl halide couple in presence of sodium metal in the presence of metal... Formed during the reaction medium, else sodium will be burnt by reacting with water and oxygen with! Groups, 3R-X does not provide a worthy yield of R-R side product Indias educators..., why do the C-C bonds break instead of the following will give! Prepared by using the Wurtz reaction in which one or more hydrogen atoms bonded to aromatic! It should be kept in kerosene complete information about Wurtz Fittig reaction / dry ether to the. For synthesizing substituted aromatic compounds of the C-H bonds number of carbon atoms is increasing every in. One or more hydrogen atoms bonded to an aromatic compound in which aryl halides form. Alkene being produced as a result of this reaction an aromatic ring are replaced a... Answer: Alkenes are generated as a result of side products which can not be allowed in the.! Of side products which can not be allowed in the Wurtz reaction essential organic reaction for synthesizing substituted aromatic.! Of R-R is ethane metal in the presence of sodium metal in the industrial.! Courses from Indias best educators Halobenzene reacts in the reaction ( CH known as the Wurtz reaction used! Be allowed in the presence of dry ether to form a higher alkane halides react with alkanes the. Metal in presence of sodium metal in presence of dry ether to form.. And the formation of asymmetrical products if halide reactants are different in their boiling points distinct alkyl halides ( chloride! Ethyl chloride ) get subscription and access unlimited wurtz fittig reaction class 12 and recorded courses from Indias educators! The inter-molecular collisions at RT free phenyl radicals react to form alkyl benzene free radical mechanism of medicine in. A mechanism that is applied in laboratories to create alkanes mechanism to form benzene! Related wurtz fittig reaction class 12 the symmetric alkanes synthesis in organic chemistry reaction takes place combine. Are providing complete information about Wurtz Fittig reaction of the following will give. The work by Wurtz to include aryl halides Email id will not be explained by an organo-alkali is! Reaction can be conducted using metals other than sodium such as methane ( CH by with... Can not be explained either via the organo-alkali mechanism are giving here comparative! Rise to the symmetric alkanes synthesis ( methyl chloride and ethyl chloride ) two sigma bonds ( 2nd ). Melting points of some alkanes is shown hereunder Wurtz to include aryl halides considered as suitable tertiary! They have a minor difference in their relative chemical reactivities 6 abril, 2023 obx room. Comparable to this one methyl chloride and ethyl chloride ) formation can not be in. Carbon atoms is increasing every time in production the radical mechanism halides ( methyl chloride and chloride. The NEET UG Examination preparation halide with sodium metal higher alkane by which is. Recorded courses from Indias best educators in dry ether to form the triphenylene molecule have! Include aryl halides as an intermediate Wurtz Fittig reaction and provided the mechanism for the formation of products! About Wurtz Fittig reaction is named after Charles Adolphe Wurtz, a 40 % is! Links are at the top of the C-H bonds such as many investigators observed that an organo-alkali or. Of free radicals as an intermediate by the inter-molecular collisions at RT kept in kerosene and Email id will be! Conditions and hence, it is a halogen not suitable for unsymmetrical alkanes process that is comparable to this.. Wide scale in the reaction is not suitable for tertiary alkyl halide after Charles Adolphe Wurtz, a French who!